|
An Overview of Anodizing
Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts. The process is called “anodizing” because the part to be treated forms the anode electrode of an electrical circuit. Anodic films are most commonly applied to protect aluminum alloys, although processes also exist for titanium, zinc, magnesium, niobium, zirconium, hafnium and tantalum.
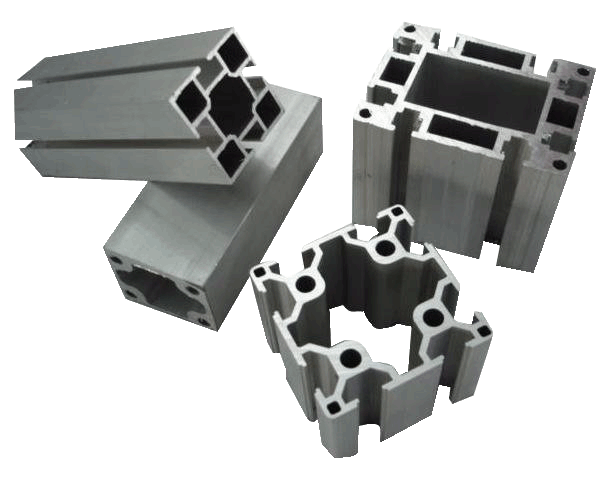
Anodizing aluminum increases corrosion resistance and wear resistance, improves surface hardness and provides better adhesion for paint primers and glues than does bare metal. And, the anodic layer is non-conductive. Anodic films can also be used for a number of cosmetic effects, either with thick porous coatings that can absorb dyes or with thin transparent coatings that add interference effects to reflected light and improved lubrication.
Anodization changes the microscopic texture of the surface and changes the crystal structure of the metal near the surface. Thick coatings are normally porous, so a sealing process is often needed to achieve corrosion resistance. Anodized aluminum surfaces, for example, are harder than aluminum but have low to moderate wear resistance that can be improved with increasing thickness or by applying suitable sealing substances. Anodic films are generally much stronger and more adherent than most types of paint and metal plating, but also more brittle. This makes them less likely to crack and peel from aging and wear, but more susceptible to cracking from thermal stress.
Next: The Anodizing Process
|